Students learn abstract ideas best when they manipulate models to find general relationships; eventually, they can create predictive rules for how the system behaves. They will understand the foundations of a concept best if they come to this understanding through their own mental effort.
This is why, when introducing chemical bonding, I refrain from lecture. Instead, the first day the students use wooden balls and sticks to build molecular models from different formulas (my Latin teacher is wincing; formulae is the true plural). Through this experience, students learn that subscripts on elements indicate the number of elements in the molecule.
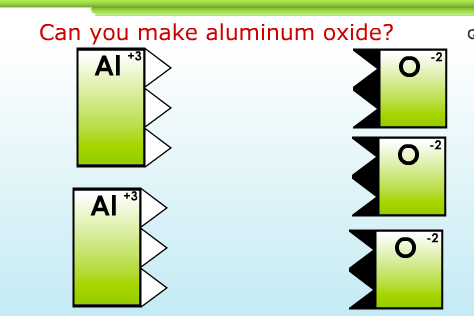
The second day, each student uses a wonderful interactive computer game to figure out for themselves how ionic compounds are written. Here’s a screenshot of the game, and a link:
I have to say, this fellow named Fletcher over at http://www.chemfiles.com/blog/ has done an outstanding job creating this and many other online simulations. Thank you, Fletcher! I hope people support you… everybody notice the Paypal donation button? 🙂
Interspersed with a few quick mini-lessons from me, my students learn a lot about chemical formulae through this series of games. Here’s my basic plan to help students understand the basics of chemical bonding:
Day 1
Teacher input to class: I remind them how to find valence electrons, and that electron-dot diagrams only include the valence. Then they’re ready to play the first game.
1. Using your periodic table, master the 22 question quiz at http://www.sciencegeek.net/Chemistry/taters/Unit3ValenceElectrons.htm
Teacher input to class: I remind them that the valence determines the number of electrons gained or lost as the atom ionizes. “Just like a table is stable if it has four legs, an atom is stable with a complete set of eight valence electrons (except Helium).” I go through a few examples on the LCD projector in the computer lab, showing them how to find the charge of ionized alkali metals, alkaline earth metals, and halogens. Then they’re ready for the second game with this last instruction: “Students, your goal is to balance the scales using the least number of atoms possible. Ultimately, you want to be able to predict the formula by just looking at the charges of the ion. Remember, overall positive charges must equal overall negative charges for a neutral, zero charged compound.”
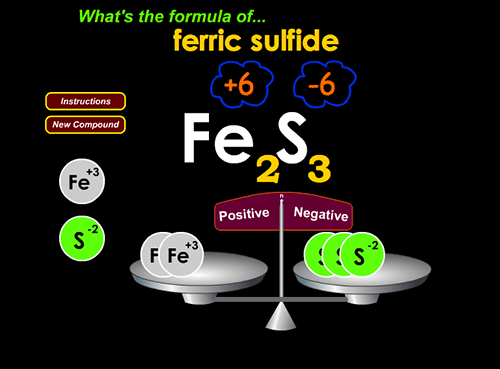
2. Write balanced ionic compound formulae at http://www.chemfiles.com/flash/formulas.html
Teacher input to class: For the next game, they need to know “sulfate”, “nitrate”, “nitrite”, “hydroxide”, & a few other polyatomic ionic compound names. I simply put up a list of ion formulae and names. It’s also helpful to point out that the positive and negative ions turn yellow when the computer recognizes you’ve matched them… if they’re not yellow, they’re not matched.
This game is valuable because it gives a graphic sense of “number of positive charges” and “number of negative charges” that need to be hooked together to balance.
3. Build ions at http://www.learner.org/interactives/periodic/groups_interactive.html
Day 2
1. Go to http://funbasedlearning.com/chemistry/chemBalancer/default.htm
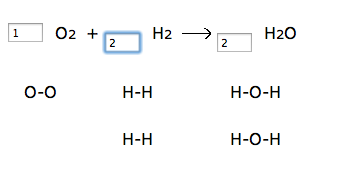
Teacher input: Make sure you’ve checked the box to “show diagrams”, and start each equation with one molecule in each box. Then try to have the same number of atoms on the left side as the right side. Complete the entire worksheet that I give you in class. The law of conservation of matter must be upheld! The number of atoms in reactants equals the number of atoms in products! I have students complete the worksheet located here: http://funbasedlearning.com/chemistry/chemBalancer/worksheet.htm
I like the chemBalancer site because it shows the number of atoms changing as students change the coefficient. Here’s a screenshot:
2. If students want a step-by-step tutorial on how to balance chemical equations, this site is helpful: http://www.wfu.edu/~ylwong/balanceeq/balanceq.html
3. Cap off a great day with a challenge! Win a MILLION BALANCES! http://www.quia.com/rr/100887.html